CrAgDb
Chaperone Repertoire in Archaeal Genomes

HSP70 System
(DnaK, DnaJ, GrpE)
HSP70 (DnaK) chaperone machinery is known to be quite important in protein folding pathways and occurs both as constitutively expressed and stress inducible forms. The first Hsp70 gene, identified by cloning and sequencing within the domain Archaea was reported in 1991, from mesophilic methanogen Methanosarcina mazei S-6 (OTG, 37°C) (Ref1). The activity of Hsp70 is associated with cofactors usually called co-chaperones. DnaK chaperone interacts with other counterparts DnaJ (Hsp40) and GrpE (Hsp24) for forming a fully functional chaperone assembly. These three proteins are ubiquitous in bacteria and eukaryotes but present in some archaeal species only. Hsp70 consists of two domains, an ATPase domain and a protein binding domain. The binding site accommodates a stretch of seven, mainly hydrophobic, amino acids in an extended conformation (Ref 2). The interaction of Hsp70 with unstructured segments of polypeptides is ATP dependent in that it binds substrates with high affinity in the post hydrolysis ADP state. The chaperone cycle can be subdivided into three steps: (1) interaction of DnaK and ATP (T state) with a hydrophobic target sequence; (2) DnaJ-triggered conversion of peptide DnaK and ATP to peptide DnaK and ADP + Pi (R state); (3) GrpE-facilitated ADP/ATP exchange and release of the polypeptide due to the binding of ATP to DnaK (Ref 3 ; Ref 4 ; Ref 5 ; Ref 6).
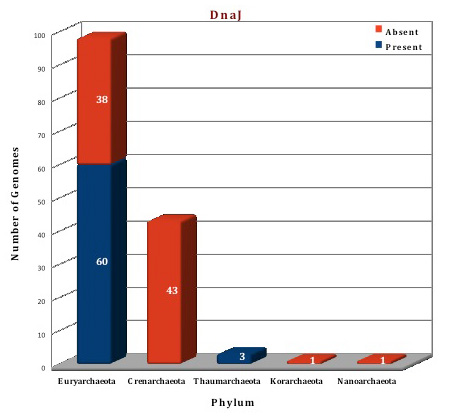
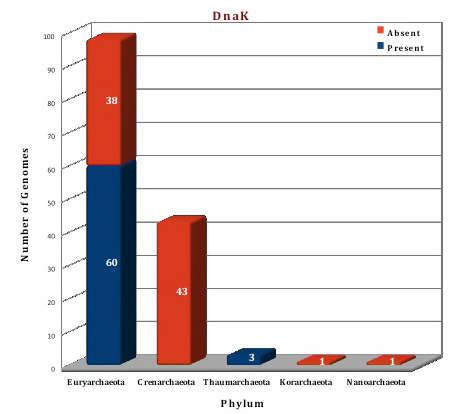
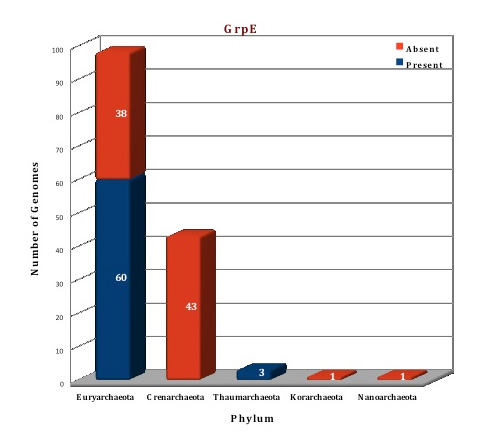
According to the data in our database, only a few members of archaeal kingdom contain Heat shock protein 70 system. The whole Hsp70 machinery is present in the orders Thermoplasmatales, Methanocellales, Methanomicrobiales, Methanosarcinales, Methanobacteriales, Nitrosopumilales, Nitrososphaerales and Halobacteriales. All components of this machinery are absent from the archaeal orders like Archeoglobales, Thermococcocales, Methanococcales, Methanopyrales, Sulfolobales, Desulforococcales, Thermoproteales, Acidilobales, Fervidicoccales and from phylum Korarchaeota, Nanoarchaeota. Thus, Hsp70 machinery is present in the genomes of 63 archaeal organisms and absent from 81 organisms. In 10 archaeal organisms (F.acidarmanus, H.hispanica, H.mukohataei, M.ruminantium, M.boonei, M.palustris, M.hungatei, M.acetivorans, M.mazei), DnaK is found in two copies while 3 related genes are found in T.archaeon. Both DnaJ and GrpE, are present as 2 copies of the gene in two organisms (M.palustris, M.boonei). No structural information is available for archaeal Hsp70 system till now but structures of some Hsp70 systems from bacterial and eukaryotic counterparts are available. Using these structures as templates, the Hsp70 (DnaK, DnaJ and GrpE) of Picrophilus torridus was modeled.
The group of DnaJ and GrpE is the main cofactors of DnaK, under stress they prevent proteins from aggregation and misfolding and can even refold aggregated proteins(Ref 7; Ref 8; Ref 9).
DnaK |
|
|
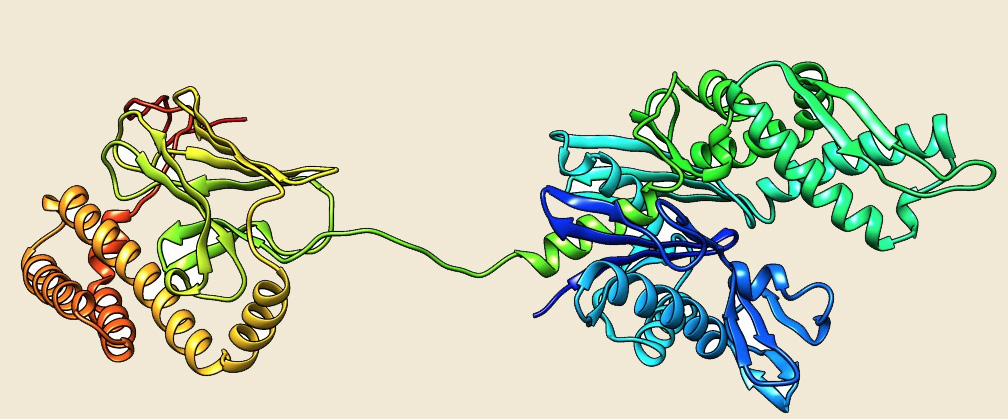
Figure: The modeled structure of Hsp70 from Picrophilus torridus. |
|
|
|
DnaJ |
|
|
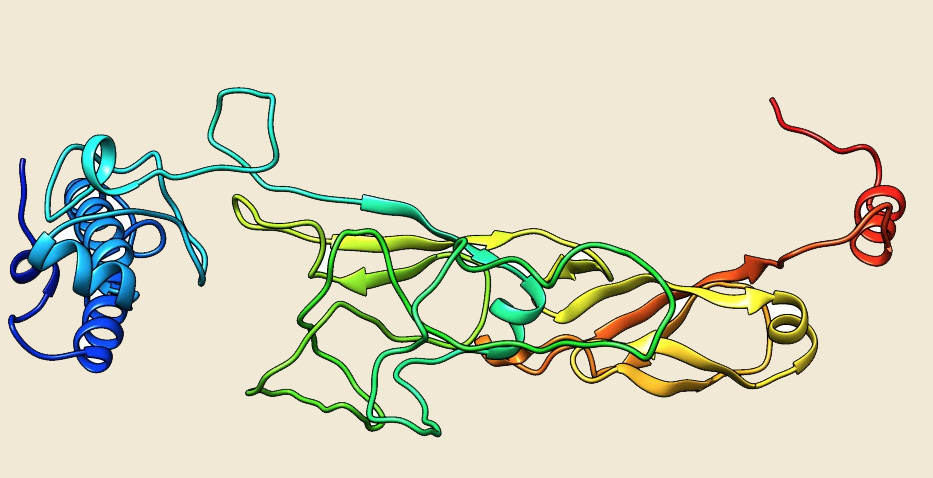
Figure: The modeled structure of Hsp70 from Picrophilus torridus. |
|
|
|
GrpE |
|
|
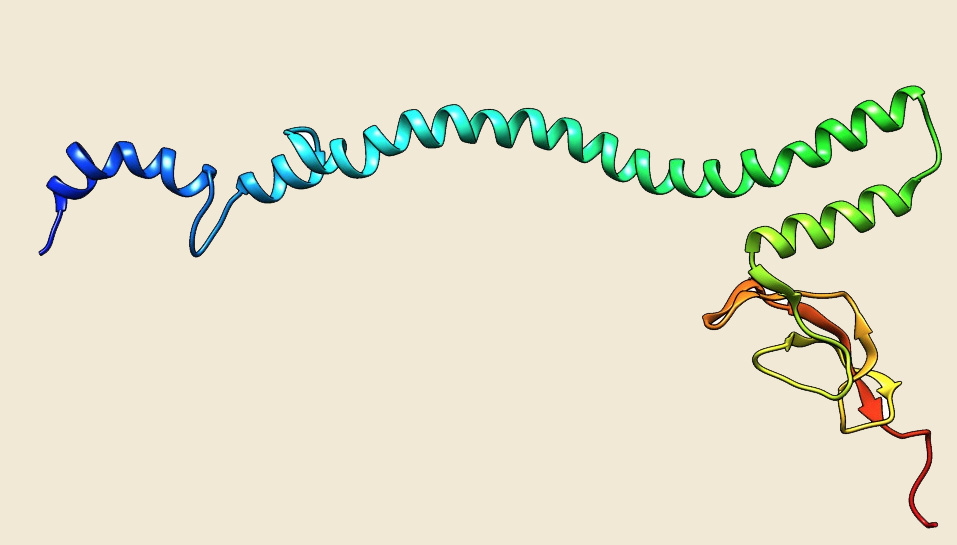
Figure: The modeled structure of Hsp70 from Picrophilus torridus. |
|
|
|
Figure: Distribution of Hsp70 chaperone proteins in 144 archaeal genomes. |
 |
 |
 |
References:
1. A dnaK homolog in the archaebacterium Methanosarcina mazei S6. ![]()
2. DnaK ATPase activity revisited ![]()
3. Structural analysis of substrate binding by the molecular chaperone DnaK. ![]()
4. The role of ATP in the functional cycle of the DnaK chaperone system.
![]()
5. The second step of ATP binding to DnaK induces peptide release.![]()
6. The power stroke of the DnaK/DnaJ/GrpE molecular chaperone system.
![]()
7. Molecular chaperones in cellular protein folding.![]()
8. Hsp70 chaperones: cellular functions and molecular mechanism.![]()
9. The Hsp70 chaperone machinery: J proteins as drivers of functional specificity.
![]()
10. Cloning of the Hsp70 gene from Halobacterium marismortui: relatedness of archaebacterial Hsp70 to its eubacterial homologs and a model for the evolution of the Hsp70 gene. ![]()
11. Cloning and characterization of a haloarchaeal heat shock protein 70 functionally expressed in Escherichia coli.![]()
12.Sequences of heat shock protein 40 (DnaJ) homologs provide evidence for a closeevolutionary relationship between theDeinococcus-thermus group and cyanobacteria.![]()
13. Evolution of a protein-folding machine: genomic and evolutionary analyses reveal lineages of the archaeal Hsp70(dnaK) gene.![]()
14. Discontinuous occurrence of the Hsp70 (dnaK) gene among Archaea and sequence features of Hsp70 suggest a novel outlook on phylogenies inferred from this protein.![]()
