CrAgDb
Chaperone Repertoire in Archaeal Genomes

NAC
(Nascent polypeptide Associated Complex)
NAC was recognized as first cytosolic chaperone that binds to the newly synthesized protein while it is still being translated and prevents aggregation/misfolding or degradation of these nascent chains (Ref 1; Ref 2; Ref 3). NAC dissociates from target proteins following the polypeptide chain release from the ribosomes (Ref 4).The cycles of binding and releasing NAC were proposed to help correct protein folding by exposing the polypeptide to the cytosol in “quantal units” rather than amino acid by amino acid, probably acting as a shield in protecting the nascent chain from inappropriate interactions with cytosolic factors.Thus NAC appears to be a general chaperone for de-novo folding, that is involved in translation and subcellular targeting of nascent polypeptides and plays a regulatory role in protein quality control (Ref 4; Ref 5; Ref 6; Ref 7). It could possibly also regulate further translation of the polypeptide through its interaction with the ribosome. NAC appears to associate with ribosomes and nascent chains in an apparent 1:1 stoichiometry through interactions of its N-terminal domain (Ref 1; Ref 8; Ref 9; Ref 10).
NAC is generally found in archaea and eukaryotes but is not present in bacteria.It is considered that NAC functions in archaea may be similar to bacterial Trigger Factors (TF) as Trigger Factor homologs are absent from all archaeal genomes. The NAC homologs contain a NAC superfamily domain, responsible for its dimerization and a C-terminal Ubiquitin Associated (UBA) domain, which takes part in the process of ubiquitination in case of eukaryotes but its role in archaeal species is unknown, since archaeal kingdom apparently lacks the ubquitination system. The Nac protein dimer resembles a six-stranded flattened barrel that exposes a number of hydrophobic residues on one of its concave surfaces (Ref 11). It is clear that hydrogen bonding in two joint β-sheets and a hydrophobic core between the two sheets are the main driving force in dimerization.This domain is structurally conserved from archaea to humans emphasizing its importance for NACs function (Ref 12).
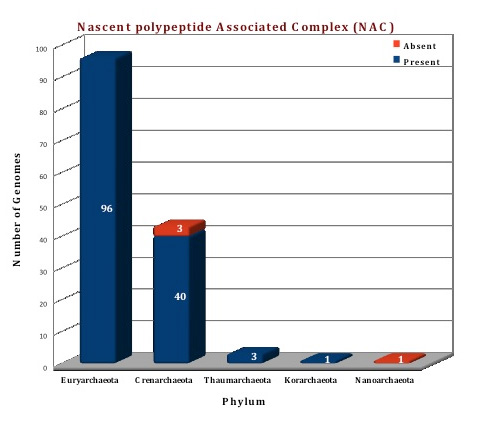
The availability of numerous completed archaebacterial genomes revealed that all archaeal taxa contain one gene with apparent homology to αNAC (Ref 13).The known crystal structure of NAC from Methanothermobacter marburgensis revealed that it is a homodimer as compared to eukaryotic NAC heterodimer.Eukaryotic NAC contains two homologous subunits, designated as α and β with apparent molecular weights of 33 kDa and 21 kDa, respectively. In archaea, a single NAC gene is found which is homologous to human αNAC and thus aeNAC exists as a homodimer. βNAC subunit does not have a C-terminal ubiquitin-associated (UBA) domain (Ref 14; Ref 15; Ref 12).Homologs of NAC protein were annotated in all the archaeal species except in 4 genomes (Nanarchaeum equitans, Sulfolobus solfataricus P2, Sulfolobus islandicus REY15A and Sulfolobus islandicus M.14.25). The crystal structure of NAC from Methanothermobacter marburgensis (1TR8) is available and we have modelled the structure of Picrophilus torridus NAC protein using 1TR8 as template.
|
|
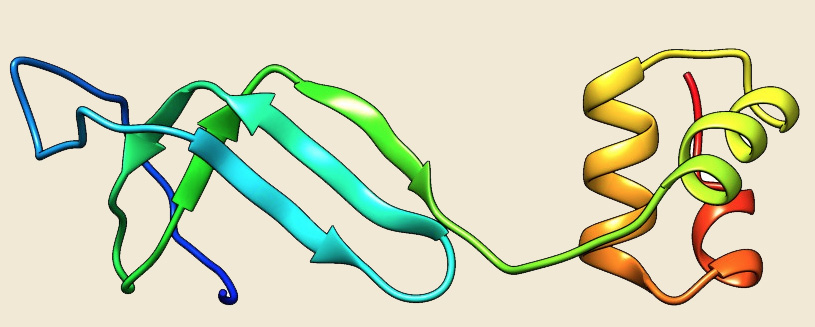
Figure: The modeled structure of NAC from Picrophilus torridus. |
|
|
|
Figure: Distribution of NAC chaperone proteins in 144 archaeal genomes. |
 |
References:
1. A protein complex required for signal-sequence-specific sorting and translocation.![]()
2. Nascent-polypeptide-associated complex: a bridge between ribosome and cytosol.![]()
3. A conserved motif is prerequisite for the interaction of NAC with ribosomal protein L23 and nascent chains.![]()
4. NAC covers ribosome-associated nascent chains thereby forming a protective environment for regions of nascent chains just emerging from the peptidyl transferase center.![]()
5. The nascent-polypeptide-associated complex: having a "NAC" for fidelity in translocation.![]()
6. The nascent polypeptide-associated complex modulates interactions between the signal recognition particle and the ribosome.![]()
7. L25 functions as a conserved ribosomal docking site shared by nascent chain-associated complex and signal-recognition particle.![]()
8. Nascent polypeptide-associated complex stimulates protein import into yeast mitochondria.![]()
9. Nascent-polypeptide-associated complex.![]()
10. Getting newly synthesized proteins into shape.![]()
11. Crystal structures of NAC domains of human nascent polypeptide-associated complex (NAC) and its αNAC subunit.![]()
12. Dual binding mode of the nascent polypeptide-associated complex reveals a novel universal adapter site on the ribosome.![]()
13. Comparative genomics of the Archaea (Euryarchaeota): evolution of conserved protein families, the stable core, and the variable shell.![]()
14. The crystal structure of archaeal nascent polypeptide-associated complex (NAC) reveals a unique fold and the presence of a ubiquitin-associated domain.![]()
15. The crystal structure of the human nascent polypeptide-associated complex domain reveals a nucleic acid-binding region on the NACA subunit .![]()
