CrAgDb
Chaperone Repertoire in Archaeal Genomes

Chaperonin-Group II
(Thermosomes)
The chaperonins of Group II, which appear to be ubiquitously present in archaeal organisms, are commonly referred to as thermosomes. The first member of this family was discovered as an ATPase complex that dramatically accumulated or elevated in response to heat shock in the hyperthermophilic archaeon Pyrodictium occultum (Ref 1). This chaperonin complex has been shown to be the most abundant protein in heat-shocked cells, comprising up to 40% of the total cellular protein (Ref 2). For the same reason, this major chaperonin with an apparent molecular mass of 60 kDa is referred to as the thermosome, having extreme thermal stability (Ref 3). The thermosome found in archaea, is more closely related to chaperonins associated with the eukaryotic cytosol (TriCC/CCT complex) than to the bacterial GroEL/ES system (Ref 4, Ref 5). Often, the designation "cct" is also used for archaeal members of Group II chaperonins, by analogy with the eukaryotic proteins. Earlier phylogenetic data analysis has shown that there are multiple "cct/thermosome" genes present in an archaeal organisms, which are likely to be redundant at some level. However, it is also possible that these individual proteins may be needed to be precisely positioned in the structural assembly complex to show particular functions (Ref 6). However, the structure of thermosome from Methanopyrus kandleri has shown the presence of only one type of subunits (Ref 7), whereas some organisms like Methanosarcina acetivorans, Methanohalophilus mahii, Natronococcus occultus, Candidatus Nitrososphaera gargensis etc. may contain upto five cct genes. Two crystal structures of thermosomes are currently available from the archaea, Thermoplasma acidophilum (1A6D) (Ref 4) and Thermococcus KS1 (1Q2V) (Ref 8), showing almost similar structures. The X-ray structure of the thermosome from T. acidophilum has been solved earlier at 2.6 Angstrom resolutions (1A6D). It consists of two eight-membered rings of alternating alpha and beta subunits, with a spherical shape; each ring has a central cavity.
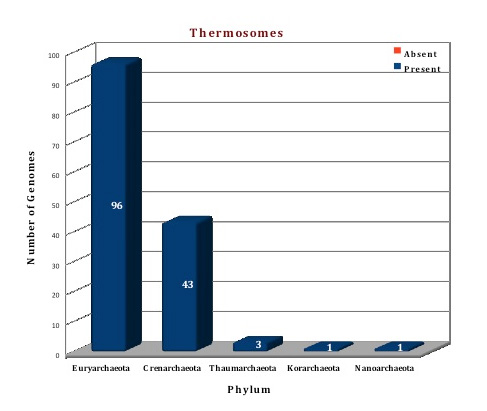
The data in our curated database suggests that thermosomes are the chaperone molecules that are present in every archaeal organism. Among the 144 archaeal genomes studied, only one gene for this protein is present in 21 genomes, suggesting that the structural compelxes here would be made up of homo-oligomers. Multiple genes coding for two, three, four and five subunits are found in 69, 34, 12 and 8 archaeal genomes respectively. The structure of two subunits, alpha and beta thermosome, from Picrophilus torridus have been modeled based on the template from Thermoplasma acidophilum.
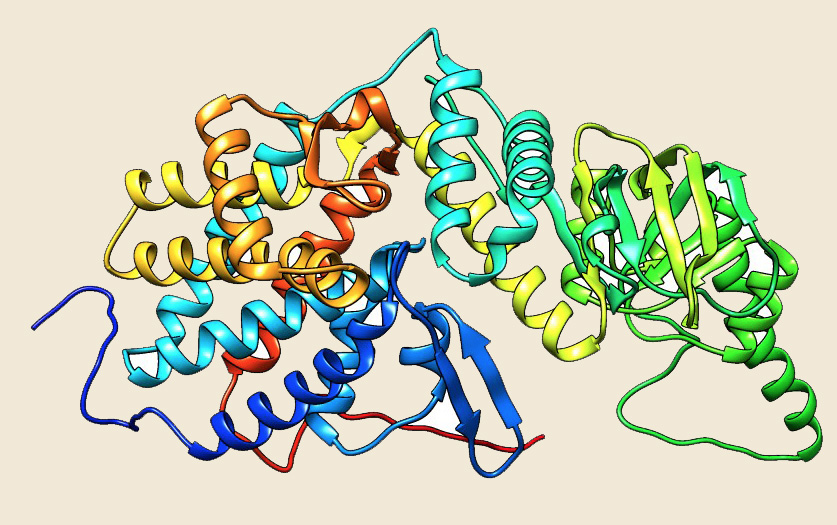 Figure: The modeled structure of Thermosome from Picrophilus torridus. |
||
|
|
Figure: Distribution of Thermosome proteins in 144 archaeal genomes. |
 |
Reference:
1.A novel ATPase complex selectively accumulated upon heat shock is a major cellular component of thermophilic archaebacteria.![]()
2. A molecular chaperone from a thermophilic archaebacterium is related to the eukaryotic protein t-complex polypeptide-1.![]()
3. Chaperonins: The hunt for the Group II mechanism.![]()
4. The thermosome: archetype of group II chaperonins.![]()
5. The Chaperones of the archaeon Thermoplasma acidophilum.![]()
6. The chaperonins: perspectives from the Archaea.![]()
7. Purification and structural characterization of the thermosome from the hyperthermophilic archaeum Methanopyrus kandleri.![]()
8. Crystal structures of the group II chaperonin from Thermococcus strain KS-1: steric hindrance by the substituted amino acid, and inter-subunit rearrangement between two crystal forms.![]()
