CrAgDb
Chaperone Repertoire in Archaeal Genomes

sHSPs
(Small Heat Shock Proteins)
Small Heat Shock Proteins (sHsps) are ubiquitous proteins, abundant in nature that are present in a wide range of organisms including archaea, bacteria and eukaryoytes (Ref1, Ref2). They function as a stress induced chaperone which generally associate with other large complex proteins and help them to fold properly. sHsps form large multimeric complexes with molecular weight ranging from 12 to 40 Kda, consisting of a large number of subunits. The sHsps share amino acid sequence homology with the 90-residue C-terminal region of vertebrate eye lens α-crystallin proteins, called the α-crystallin domain or small-heat-shock-protein domain, which is conserved in this family of protein through all domains of life. Both alpha-crystallins and sHSPs are known to function as molecular chaperones having the ability to prevent aggregation of denatured proteins under stress conditions (Ref 3; Ref 4; Ref 5; Ref 6; Ref 7). In sHsps, the α-crystallin domain (80-100 amino acid residues) is the middle domain and is preceded by a highly variable N-terminal region and is followed by a short, poorly conserved C-terminal extension (Ref 8; Ref 9). The α-crystallin domain thus represents the signature motif of sHsps.
All members of sHsp family have a conserved structure, comprising of a compact β-sheet sandwich similar to the immunoglobulin-like fold. α-crystallin domains can dimerize through the formation of an intersubunit composite β-sheet, and this dimerization is a conserved feature of sHsps. The most conserved motif of the alpha-crystallin-type HSPs (AxxxxGvL) (Ref 8) has also been observed in all of the sHSPs from extremophiles (Ref 10). In all crystal structures, the C-terminal region following the α-crystallin domain is involved in stabilizing the dimerization complex (Ref 11). The crystal structure of the sHsp in Methanocaldococcus jannaschii is a 16.5 kDa protein that forms a unique spherical oligomer of 24 subunit homooligomeric arrangements into a hollow sphere with multiple pores.
According to the data of our database, the copy number of Hsp genes is variable among different archaeal species. All completely sequenced archaeal genomes contained minimum one and a maximum of five members of sHsp family. Thermophilic and hyperthermophilic archaea contain one, two or three Homologs. All the halobacteriales have five members and Thermoplasmatales have two sHsps. The sHsps molecules seem to be very flexible, as they are resolved only partially in the available crystal structures.Twocrystal structures of sHsps from Methanocaldococcus jannaschii (1SHS)(16.5 kDa) and Sulfolobus tokodaii (3VQK)(14.0 kDa) are currently available (Ref 10; Ref 12). The three dimensional structure of two small heat shock proteins from Picrophilus torridus was modelled using Sulfolobus tokodaii as a template
|
|
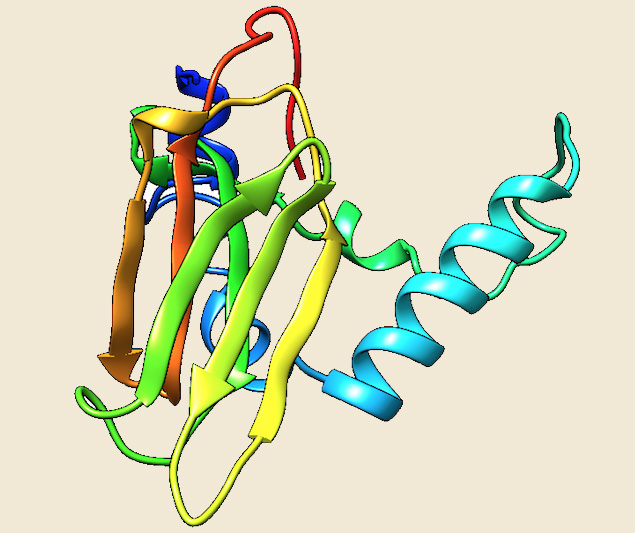
Figure: The modeled structure of sHSP from Picrophilus torridus. |
|
|
|
Figure: Distribution of sHSPs chaperone proteins in 144 archaeal genomes. |
 |
References:
1. Molecular chaperones: small heat shock proteins in the limelight. ![]()
2. Molecular chaperones: containers and surfaces for folding, stabilising or unfolding proteins.![]()
3. Alpha-crystallin can function as a molecular chaperone.![]()
4. Small heat shock proteins are molecular chaperones.![]()
5. ATP-enhanced molecular chaperone functions of the small heat shock protein human alphaB crystallin.![]()
6. Site-directed mutations within the core "alpha-crystallin" domain of the small heat-shock protein, human alphaB-crystallin, decrease molecular chaperone functions.![]()
7. sHsps and their role in the chaperone network.![]()
8. The expanding small heat-shock protein family, and structure predictions of the conserved "alpha-crystallin domain".![]()
9. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides.![]()
10. Small heat shock protein of Methanococcus jannaschii, a hyperthermophile.![]()
11. Crystal structure and assembly of a eukaryotic small heat shock protein.![]()
12. Structural studies on the oligomeric transition of a small heat shock protein, StHsp14.0.![]()
