CrAgDb
Chaperone Repertoire in Archaeal Genomes

Prefoldin
(Prefoldin α & β)
Prefoldin/GimC are chaperones that are known to universally present in eukarya and archaea, but are absent in bacteria (Ref 1). They form a heterohexameric complex that captures an unfolded protein and transfers it to group II chaperonin for the completion of folding (Ref 2; Ref 3; Ref 4). The transfer of a substrate from prefoldin to chaperonin is known to be mediated through the prefoldin β subunit. It is now assumed the prefoldin alone is unable to refold the denatured proteins. They appear to be holdases type of chaperones, showing no ATPase activity. Prefoldins can be grouped into two main classes, α and β, that appear to be evolutionary related. The archaeal organisms posses one or two members of each class whereas eukaryotic organisms have two subunits of α and four subunits of β class. Prefoldins are known to be involved in cotranslational folding of cytoskeletal proteins like actin and tubulin in eukaryotes. Although actin or tubulin are not present in archaea, both prefoldin homologues and group II chaperonins have invariably been proposed to be involved in stabilizing a wide variety of other unfolded proteins (Ref 2; Ref 5).
The genome analysis of archaea shows that thermosomes found in different archaea are closely related, some are homo-oligomers (Ref 7), while others are hetero-oligomers containing one to three cct genes, whereas some organisms like Methanosarcina acetivorans, Methanohalophilus mahii, Natronococcus occultus, Candidatus Nitrososphaera gargensis etc. may contain upto five cct genes. In the thermophilic archaeon, the chaperonin complex has been shown to be the most abundant protein in heat-shocked cells, comprising up to 40% of the total cellular protein (Ref 8). For this reason it is referred to as the thermosome, having extreme thermal stability (Ref 9), although the designation cct is also used by analogy with the eukaryotic proteins.
The first crystal structure of prefoldins was from the archaeon Methanothermobacter thermautotrophicus. The protein complex has a jellyfish like structure, with a globular body consisting of six canonical, antiparallel coiled coils containing two α and four β subunits with their N and C domains oriented outwards from an oligomerization domain (Ref 4). Both N and C terminal regions of the β subunit are important for its molecular chaperone activity. The archaeal prefoldin complex is rich in α-helical regions and contains extensive coiled coil regions. There is evidence that coiled coil regions having partially exposed hydrophobic regions that may be required for the multivalent binding of non native proteins.
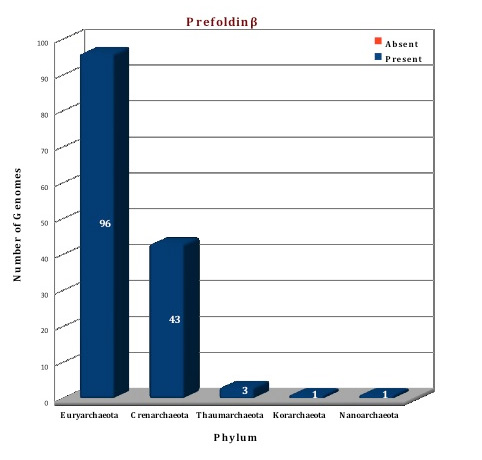
According to our database collection, a single copy of the prefoldin β subunit is present in all the 144 archaeal genomes, whereas prefoldin α subunit is present in 142 archaeal genomes, being absent in two genomes (I.aggregans, N.equitans). In nine archaeal species (I.hospitalis, M.infernus, M.jannashii, M.sp.FS406-22, M.vulcanius, T.gammatolerans, T.kodakarensis, T.sp. AM4, T.sp.CL), the prefoldinα is present in two copies, which results in a total 151 sequences. Three crystal structures of the prefoldin complex are currently available, from Methanothermobacter thermoautotrophicus (1FXK), Pyrococcus horikoshii (2ZDI) and Thermococcus strain KS1 (2ZQM) (Ref 4; Ref 6). The structure of both the subunits from Picrophilus torridus were modelled on the basis of the template from Methanothermobacter thermoautotrophicus.
PrefoldinA |
|
|
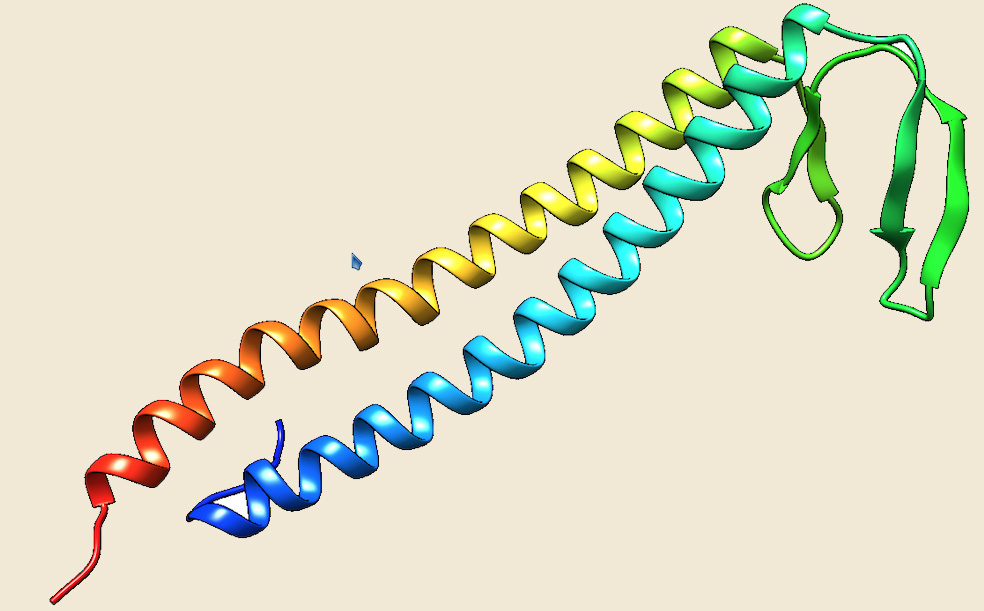
Figure: The modeled structure of PrefoldinA from Picrophilus torridus. |
|
|
|
PrefoldinB |
|
|
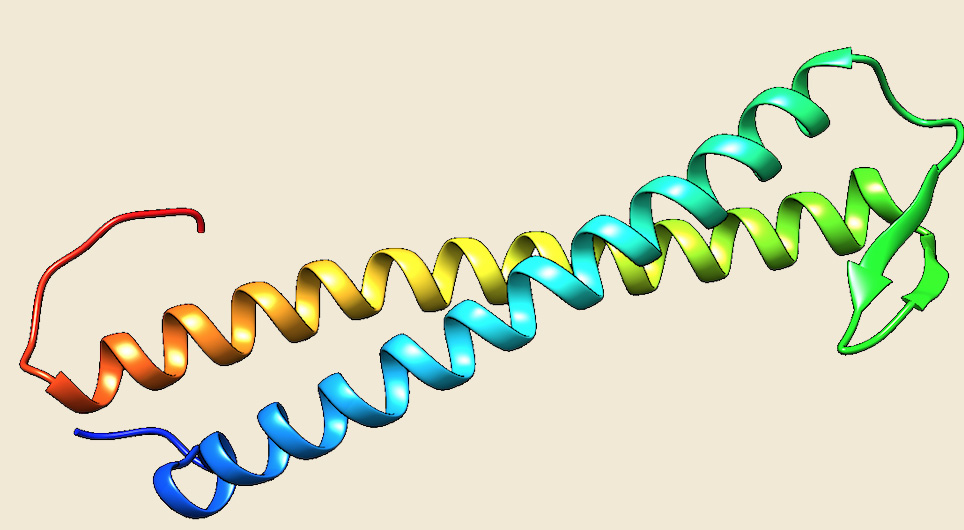
Figure: The modeled structure of PrefoldinB from Picrophilus torridus. |
|
|
|
Figure: Distribution of Prefoldin chaperone proteins in 144 archaeal genomes. |
 |
 |
References:
1. Evolution of assisted protein folding: the distribution of the main chaperoning systems within the phylogenetic domain archaea.![]()
2. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin.![]()
3. Molecular chaperones in the cytosol: from nascent chain to folded protein.![]()
4. Structure of the molecular chaperone prefoldin: unique interaction of multiple coiled coil tentacles with unfolded proteins.![]()
5. MtGimC, a novel archaeal chaperone related to the eukaryotic chaperonin cofactor GimC/prefoldin.![]()
6. Structure and molecular dynamics simulation of archaeal prefoldin: the molecular mechanism for binding and recognition of nonnative substrate proteins. ![]()
